July 5 2011
2
05
/07
/July
/2011
13:10
I have compiled a list of sources for the elements that are available to the amateur chemist. Chromium will be discussed here.
Chromium is an extremely shiny bluish-silvery metal in pure form. It is commonly found as a thin plating on objects known as a chrome plating. Chromium(III) compounds are necessary to life in small amounts, while chromium(VI) compounds are highly toxic. Chromium forms a passivation layer like aluminium, allowing it to keep its shine.
In element form: Reduce chromium(III) compounds or chromium(VI) compounds by electrolysis. Find a chromium or chrome plated object. Stainless steel has about 18% chromium in it.
In compound form: Chromate conversion coated hardware contains a small layer of chromates on the surface to prevent corrosion. Chrome oxide green and chrome rouge are both composed of chromium(III) oxide. Chromated copper arsenate wood contains chromium as well. Many cassette tapes have chromium dioxide in the tape.
Here is my sample of chromium. It is a chrome plated Toyota symbol.

Published by LanthanumK
-
in
Elements
July 4 2011
1
04
/07
/July
/2011
13:41
Warning: This experiment deals with corrosive substances and toxic fumes. Work outside or in a fume hood.
You will need:
Sulfur chloride mixture
Water
Sulfur
The sulfur chloride mixture created in a previous experiment should be orange or red. Add some sulfur to it when warm. The sulfur should dissolve and react with the red sulfur dichloride to make yellow disulfur dichloride. This sulfur chloride is more stable. Disulfur dichloride dissolves sulfur as well. Add disulfur dichloride to water. A yellow solid is formed, along with release of sulfur dioxide gas and hydrogen chloride fumes.
Published by LanthanumK
-
in
Experiments
July 4 2011
1
04
/07
/July
/2011
13:29
I have compiled a list of sources for the elements that are available to the amateur chemist. Vanadium will be discussed here.
Vanadium is a bluish gray metal. It is rarely found as a pure metal but much more commonly found as an alloy with steel. Vanadium compounds are used as catalysts and vitamin supplements. Vanadium is a moderately toxic transition metal. Some sea creatures contain large amounts of vanadium.
In element form: Vanadium steel contains from 0.1 to 5% vanadium in it.
In compound form: Vanadyl sulfate is used in vitamin supplements. Bismuth yellow is made of bismuth vanadate.
Here is my sample of vanadium. It is a screwdriver blade composed of vanadium steel.

Published by LanthanumK
-
in
Elements
July 2 2011
6
02
/07
/July
/2011
13:34
Warning: This is a rather advanced experiment that requires the use of toxic gases and highly reactive and corrosive liquids. This procedure must be done in a fume hood or outside. Research thoroughly before completing. This experiment has not been verified by the author.
You will need:
Sodium hypochlorite (bleach)
Hydrochloric acid
Concentrated 98% sulfuric acid
Sulfur
Glass tubing
Stoppers
Erlenmeyer flasks
Sodium hydroxide
Source of heat
Dry ice
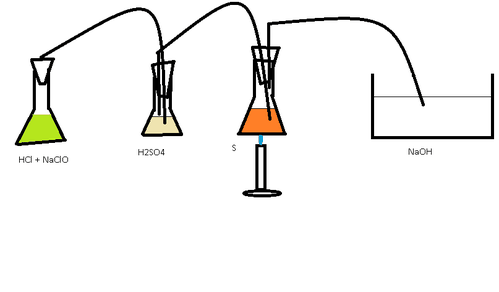
First, construct your apparatus. Flask 1 will have a tube running from it into Flask 2. Flask 2 will have a tube running from it to Flask 3. Flask 3 will have a tube running from it to Tub 1. Tubes are made of glass, but the connecting tubes can be made of rubber.
Add sodium hypochlorite to Flask 1. Add sulfuric acid to Flask 2 so that one tube is under the acid while the other is above. Add sulfur into Flask 3 with a small amount of dry ice. Add sodium hydroxide solution to Tub 1 and place the tube under the solution. Then, begin gently heating Flask 3. Because of the carbon dioxide, the sulfur should not ignite. It should instead melt into a light yellow thin liquid. Make sure one of the glass tubes is in the liquid sulfur. Keep the sulfur temperature low enough so that the glass tube does not get stuck in highly viscous hot molten sulfur. Then, add a small amount of hydrochloric acid to the bleach solution in Flask 1. You should get some bubbling in Flask 2, where the sulfuric acid dries the chlorine gas. The chlorine gas should then bubble into the sulfur, producing a chemical reaction to form disulfur dichloride if there is a little chlorine and sulfur dichloride if there is much chlorine. Continue adding hydrochloric acid and bleach, if necessary, to Flask 1 until the molten sulfur has become transparent but not clear. Then, wait for the sulfur chloride to cool. You should have formed a mixture of sulfur dichloride and disulfur dichloride.
Published by LanthanumK
-
in
Experiments
July 2 2011
6
02
/07
/July
/2011
13:08
I have compiled a list of sources for the elements that are available to the amateur chemist. Titanium will be discussed here.
Titanium is a light silvery metal. It is very strong and quite expensive. It is reactive but protected by a durable transparent oxide film. When titanium electrolytically oxidized, the oxide film becomes thicker and starts diffracting light, producing colored titanium. Titanium forms sparks when ground and burns in both air and nitrogen when heated. Its nitride is unusually stable. Titanium tetrachloride is a fuming liquid that is used to make smoke.
In element form: Some hardware like bolts and washers are made of pure titanium. Expensive scissors are either titanium plated or made with a titanium alloy.
In compound form: Titanium dioxide is a common white pigment. Piezocrystals can contain lead titanate (if yellow) or lead titanate zirconate (if gray).
Here is my sample of titanium. It is a piece of foil from GalliumSource.

Published by LanthanumK
-
in
Elements
July 1 2011
5
01
/07
/July
/2011
21:43
Published by LanthanumK
-
in
Experiments
July 1 2011
5
01
/07
/July
/2011
14:36
I have compiled a list of sources for the elements that are available to the amateur chemist. Scandium will be discussed here.
Scandium is a slightly yellowish silvery metal. When alloyed with aluminium it makes aluminium very strong. Scandium is rare as an element and very expensive. It was used by the Russians in military aircraft. Scandium does not have very interesting chemistry.
In element form: Some bicycle frames and other expensive metal objects are made of scandium-aluminium alloy, which contains from 0.1-0.5% of scandium metal.
In compound form: Scandium iodide is used in metal halide lamps.

Published by LanthanumK
-
in
Elements
June 30 2011
4
30
/06
/June
/2011
16:48
Warning: Sulfuric acid at these concentrations is slightly corrosive. Be careful when handling. Sulfur dioxide fumes are poisonous. Do not inhale them. Complete this experiment in a well-ventilated area.
You will need:
Sulfur
Hydrogen peroxide, 3% (a greater percentage gives more concentrated sulfuric acid)
Glass container
Aluminium foil
Shape the aluminium foil into a tray. Fill it with sulfur. Ignite the sulfur by heating the tray over a flame. Place it in the container and close the lid. The jar will fill with sulfur dioxide and sulfur fumes. Open the jar, remove the tray, and add some hydrogen peroxide. Shake the jar. Reignite the sulfur and repeat the process until all of the sulfur has burnt. Now you have a dilute sulfuric acid solution. If you want it more concentrated, boil it down.
Published by LanthanumK
-
in
Experiments
June 30 2011
4
30
/06
/June
/2011
14:39
I have compiled a list of sources for the elements that are available to the amateur chemist. Calcium will be discussed here.
Calcium is a somewhat soft, silvery-gray metal. It reacts with water to produce white calcium hydroxide and hydrogen gas. Calcium compounds are very common in the earth. Calcium carbonate is found in calcite and mollusk shells. Calcium phosphate is found in bones. Calcium as a metal has fewer uses.
In element form: Some lead acid batteries use a 0.1% calcium alloy.
In compound form: Calcite, aragonate, and mollusk shells contain calcium carbonate. Bones are made of calcium phosphate.
Here is my sample of calcium metal. It was purchased for $13.00 from GalliumSource.

Published by LanthanumK
-
in
Elements
June 29 2011
3
29
/06
/June
/2011
14:27
Warning: Sulfur compounds can be irritating. Do not inhale them. It is best to do this reaction in a well-ventilated place. Heat is released during this reaction. Keep flammables away.
You will need:
Sulfur (flowers of sulfur)
Pliers
Aluminium foil
Glass jar
Container
Ammonia solution
I know that sulfites can be obtained easily, but they can also be made from sulfur, which can also be obtained easily. First, shape the aluminium foil into a shallow pan. Fill it with sulfur powder. Holding it with pliers, heat it in a flame. It will melt, then ignite. As soon as you see flames, place it in the glass jar and secure the lid. It will burn until the oxygen is depleted and then go out, leaving yellow-white sulfur fumes floating around. Open the jar and carefully pull out the foil pan. Add some ammonia. Close the jar and slosh around. Pour the ammonia solution into another container. Reignite the sulfur and repeat the process until all of the sulfur has burned. You will be left with a more or less basic ammonium sulfite solution. Evaporate it. Excess ammonia will leave, and crystals of ammonium sulfite will form. If you use sodium hydroxide instead of ammonia, you will need to measure it or you will get sodium hydroxide mixed with your sodium sulfite. To test ammonium sulfate, place it in a basic solution. Ammonia gas should be given off. Place it in an acidic solution. Sulfur dioxide gas should be given off. If you did not use enough ammonia, you may form ammonium bisulfite.
Published by LanthanumK
-
in
Experiments








