Buffers are used to regulate pH. They contain a weak acid and its conjugate base (as in this case), or a weak base and its conjugate acid. When either a base or may acid is added to the solution, it neutralizes one of the constituents to form water. This is why it resists changes in pH. Buffers are important in your body. Your blood pH has to stay in a very narrow range (7.35-7.45). If the pH falls below 6.8 or rises above 7.8, serious injury may occur, even resulting in death. Buffers protect your blood when it is barraged by metabolic wastes or foods.
The buffer I used here is acetic acid with sodium acetate. It contains a weak acid (acetic acid) along with its conjugate base (acetate ion). I added a 0.015 M (very dilute) NaOH (sodium hydroxide) solution to the solutions which contained phenolphthalein indicator, which is colorless in pH<8.3 and red in increasing brilliance to pH 10, which is the end point. One solution contained the buffer, and one didn't.
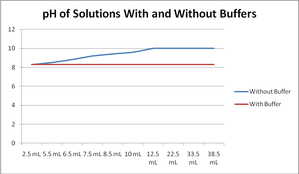
The lack of pH change with the buffer is very clear.
Sodium acetate with acetic acid was the buffer used in my Chem II lab on buffers. The pH change when some HCl was added was -0.29. By comparison, when water was used, it was -3.5. The pH change when some NaOH was added to the buffer was +0.28. The pH change when some NaOH was added to water was +6.67.


